About Us
Nestal BioSciences Inc. is dedicated to revolutionizing the medical sector by developing and commercializing cutting-edge innovations that enhance patient outcomes, improve quality of life, and create value for stakeholders. We strive to be a leader in the industry by driving innovation and leveraging strategic partnerships and collaborations to deliver exceptional value to patients, healthcare providers, and shareholders.
Our mission is to revolutionize the healthcare segments we engage in by providing innovative, safe, and affordable solutions that mitigate health risks and enhance patient quality of life. We empower healthcare providers and payers with the knowledge and tools needed to achieve positive outcomes. We are committed to collaboration, innovation, and excellence to drive meaningful change in healthcare.
Our vision is to lead the way in delivering innovative and effective healthcare solutions, empowering patients and improving outcomes. We aim to make the highest quality care accessible to everyone, enabling faster and more effective interventions worldwide. Our cutting-edge technology and unwavering commitment to excellence are paving the way for a brighter future in healthcare.
ClearOne™
ClearOne™ is poised to transform the landscape of cytology testing by making high-quality, reliable cancer screening accessible to women everywhere. Its innovative approach addresses the key barriers of cost, equipment dependency, and geographic limitations with delivering outstanding results, ultimately contributing to better health outcomes on a global scale.
ClearOne™ Digital and AI Options
ClearOne™ Digital and AI Options
ClearOne™ is set to integrate advanced digital capabilities, enabling labs to upload prepared cytology slides for easier access by qualified experts from anywhere in the world. This integration ensures that even in regions where local cytology experts are unavailable, credentialed professionals can remotely review slides, providing expert analysis and enhancing diagnostic accuracy.
The AI-driven platform will support clinical decision-making by analyzing digitized images, helping lab professionals deliver faster and more precise results. This seamless integration of digital capabilities will allow ClearOne™ to be deployed in diverse environments—without the need for costly equipment—making it adaptable to both high-resource and underserved areas.
Key Benefits:
- Integrated Digital Slide Upload: Global access for expert review of prepared slides.
- AI-Supported Clinical Decisions: Enhances precision and speed in diagnostics.
- No Expensive Equipment Required: Easy deployment across diverse settings.
- Global Expertise: Credentialed experts can provide input from anywhere.
ClearOne™ will empower labs with cutting-edge technology, driving better outcomes and expanding access to cervical cancer screening worldwide.
BETTER
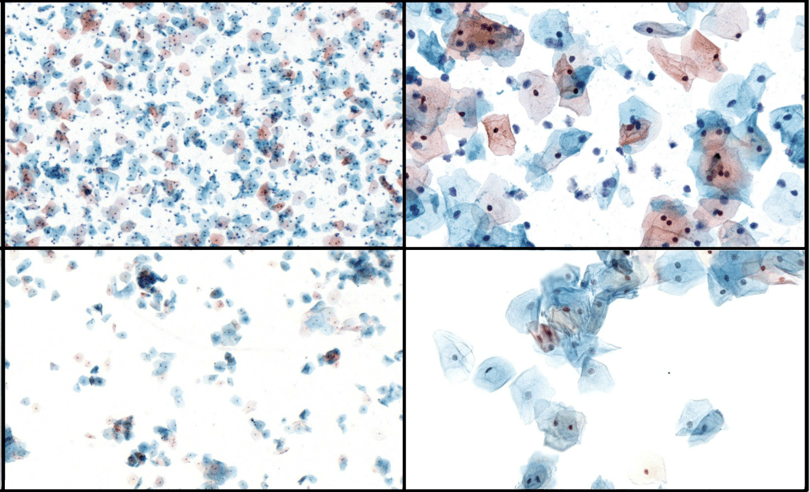
ClearOne™ liquid cytology test produces superior single layer slides with lower UNSAT rates than traditional pap test. It also produces lower UNSAT rates than other liquid-based cytology tests.
FASTER
ClearOne™ test requires minimal training and produces higher number of slides* in a given hour compared to specialized equipment used for slide preparation.
AFFORDABLE
ClearOne™ is competitively priced for any market and does not require use or purchase of any special equipment while providing limited resource countries with a superior test anywhere in the world and be up and running in a few hours.
Use Cases
ClearOne™ is well developed for cytology testing needs including cervical, oral, uterine, and bladder cancer screening. This advanced technology produces high-quality, true mono-layer slides without the need for specialized equipment, making it an ideal solution for diverse clinical applications in any country, worldwide.
PARTNERS AFFILIATIONS ASSOCIATIONS








Team
Leadership and Operations: The leadership team with over 20 years of experience in healthcare sales and marketing in therapeutics and diagnostics and developed key influencers to drive sales across hospitals, clinics, and long-term care facilities, with significant experience in launching new products that generated over $1 billion in revenue.
Strategic and Legal: The strategic, legal, and regulatory experts are skilled in life sciences M&A, venture capital, and financing. They ensure compliance with FDA, EUA, CE, and other manufacturing standards, and maintain resilience networks within U.S. government agencies and international organizations.
Scientific and Clinical Advisors: The scientific advisory team includes seasoned professionals with over 25 years of experience in cancer cytology and clinical laboratory operations. Their expertise extends to advising the WHO and directing labs at leading institutions.
Regulatory Expertise: A dedicated regulatory expert with over 20 years of experience ensures adherence to all relevant regulations, facilitating smooth and compliant operations.
Financial Operations: The financial operations are overseen by a Certified Public Accountant with extensive financial, operations, and management experience within diagnostics, ensuring robust financial planning and operations.
Contact Us
Wilmington DE Las Vegas NV ASIA
info[at]nestalbio.com
